How would you find the average atomic mass of sulfur from the following data:S-32 95.002% (31.972), S-33 0.76% (32.971), S-34 4.22% (33.967), and S-36 0.014% (35.967)?
1 Answer
Explanation:
3ds to cia converter godmode9. The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances.
In your case, the average atomic mass of sulfur will be calculated using the given atomic masses of its four isotopes and their respective decimal abundance, which is simply the percent abundance divided by
So, you know that you have

The atomic mass and abundance of Si-28 is 27.977 amu and 92.2%. The atomic mass and abundance of Si-29 is 28.976 amu and 4.7%. A sulfur atom contains 16 protons. Sulfur is created in huge stars and is present in various kinds of meteorites. It is produced during fusion reaction between nucleus of helium and silicon. In the Earth’s crust, sulfur is the 5 th most abundant element by mass. It is ubiquitous in volcanic regions and in hot soring areas of the world.
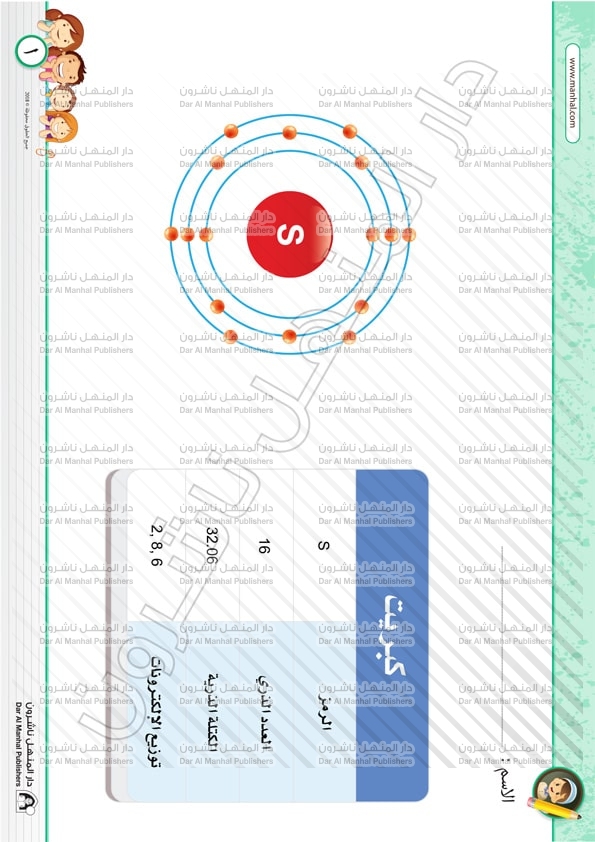
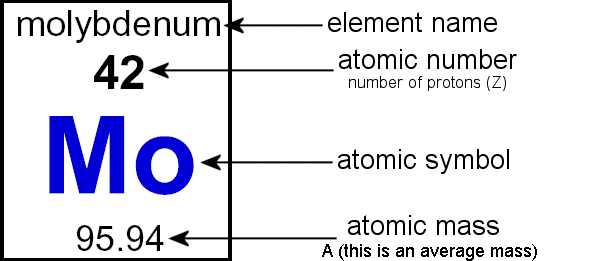

This means that the average atomic mass of sulfur will be
Sulfur Atomic Mass Number
Sulfur 32 Atomic Mass
Sulfur Atomic Mass Grams
Related questions

